
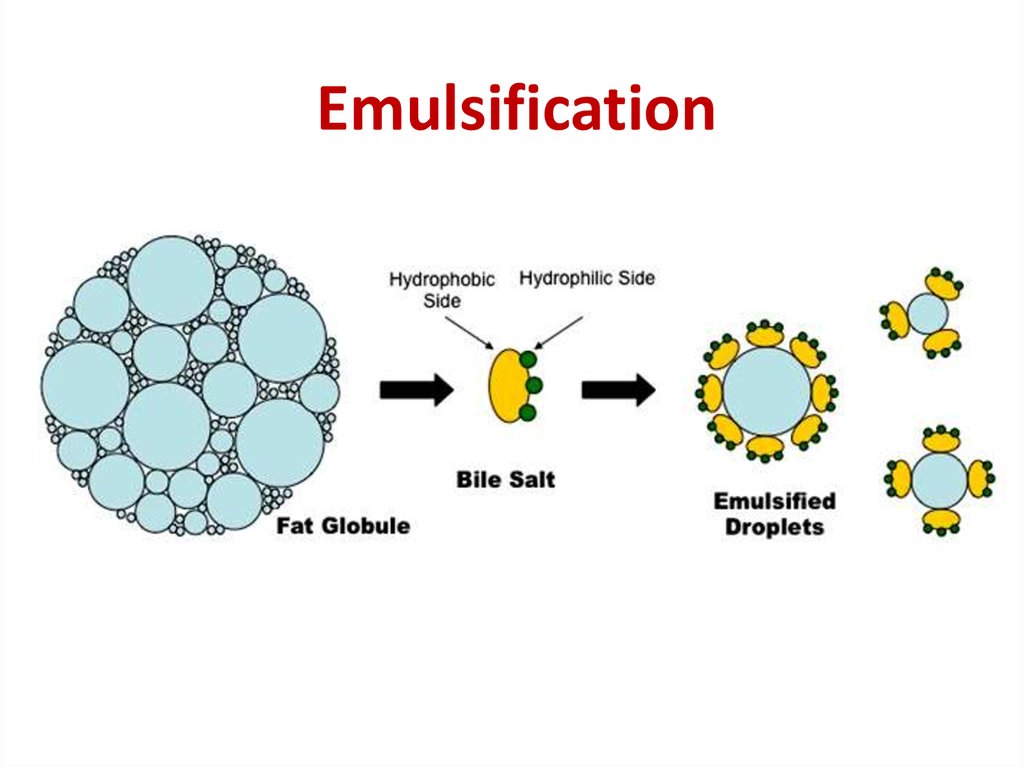
The ability of an emulsion to retain its properties over time is known as the stability of an emulsion. Viscosity modification – Certain emulgents such as acacia, tragacanth, carboxymethylcellulose, polyethylene glycol, etc increase the viscosity of the medium, which helps create and maintain the suspension of globules of the dispersed phase.This repulsive force causes them to remain suspended in the dispersion medium Repulsion theory – The theory proposes that the emulsifying agent creates a film over one phase that forms globules, which repel each other.Surface tension theory – According to this theory, emulsification takes place by the reduction of interfacial tension between two phases.Examples include egg yolk, sodium phosphates, sodium stearoyl lactylate, etc Mechanisms of EmulsificationĪ number of different chemical and physical processes and mechanisms can be involved in the process of emulsification. For example, proteins dissolve better in water than in oil, and so tend to form oil-in-water emulsions (that is, they promote the dispersion of oil droplets throughout a continuous phase of water).Įmulsifiers that are more soluble in water generally form oil-in-water emulsions, while emulsifiers that are more soluble in oil will form water-in-oil emulsions. Because of this, emulsifiers tend to have more or less solubility either in water or in oil.Īccording to the Bancroft rule, Emulsifiers and emulsifying particles tend to promote dispersion of the phase in which they do not dissolve very well. water-soluble) part and a non-polar (i.e. It acts on the interface and increases the kinetic stability of an emulsion so that the size of the droplets does not change significantly with time, thus stabilizing the emulsion.Įmulsifiers are compounds that typically have a polar or hydrophilic (i.e. These are surface active agents that are added to the emulsions to stabilize the two phases. When kept for longer periods of time or in case of absence of an emulsifying agent, the phases in the emulsion tend to separate, resulting in “cracking of emulsion” or ” phase inversion”.Emulsions are prepared by continuous mixing or agitation of the two phases.Emulsions are highly unstable systems and require an emulsifying agent or emulsifier ( These are usually surface active agents also known as “surfactants”).Emulsion particles unavoidably form dynamic inhomogeneous structures on small length scale.Since these systems have a particle size less than 100 nm, they have a translucent appearance. In case of special classes of emulsions like nanoemulsions and microemulsions, the system appears translucent in colour. This property is due to the fact that light waves are scattered by the droplets only if their sizes exceed about one-quarter of the wavelength of the incident light.

If the emulsion is concentrated enough, the colour will be distorted to comparatively longer wavelengths and will appear more yellow. This phenomenon is known as the “Tyndall Effect”. In case of dilute emulsions, a low-wavelength light will be scattered more, and the emulsion will appear blue in colour. Emulsions appear white when all light is scattered equally. The color of the emulsion depends upon its concentration. The emulsions tend to have a cloudy appearance because the phase interphases present in the emulsion scatter light at different wavelengths. Relationship Between Concentration and AppearanceĪ boundary that exists between the dispersed phase and continuous phase is known as the interface. Whether an emulsion of oil and water turns into a “water-in-oil” emulsion or an “oil-in-water” emulsion depends on the volume fraction of both phases and the type of emulsifier used to emulsify them. If the aqueous phase is the dispersed phase and the oil phase is the continuous phase, then its known as “water in oil” If the oil phase is dispersed in a continuous aqueous phase the emulsion is known as “oil in water”. The type of emulsion depends upon the properties of the dispersed phase and continuous phase. Browse more Topics under Surface ChemistryĮmulsions can exist as “oil in water” or ” water in oil” of emulsions. Examples of emulsions include mayonnaise, milk, lotions, etc. Emulsions are part of a more general class of two-phase systems of matter called colloids.Īlthough the terms colloid and emulsion are sometimes used interchangeably, the term emulsion is used only when both the phases are in a liquid state. These phases are present in such as way that one phase is dispersed in the other continuous phase. Emulsions are biphasic liquid systems consisting of two immiscible liquid phases.


 0 kommentar(er)
0 kommentar(er)
